Introduction
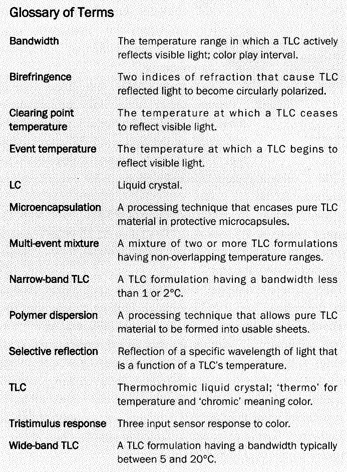
| The development of liquid crystal (LC) based thermography overthe past 30 years has provided thermal engineers with a relatively inexpensivetechnique for visualizing and measuring surface temperature. Engineers andscientists have successfully used LC thermography to investigate various thermalphenomena in a wide variety of applications. These applications includeelectronics cooling (Figures 1 and 2), gas turbine heat transfer, boiling heattransfer, and fluid temperature measurement. Commercially available,quantitative LC thermography systems have recently begun to emerge from thelaboratory into the thermal engineering community. The use of modern high speedcomputers, solid-state color cameras and image digitizers has helped thisemerging trend tremendously (see Figures 2 and 3). In order to properlyinvestigate thermal phenomena with LC thermography, a basic understanding of thedifferent types of LC materials is necessary. | 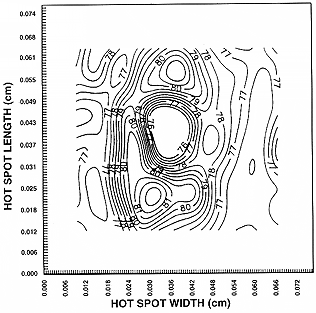 Figure 1. Temperature contour plot of a ‘hot-spot’ on a thin film resistor. From [1], bypermission. |
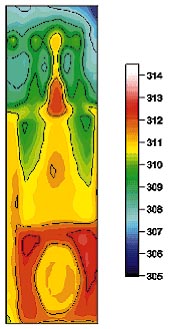 Figure 2. Temperature distribution on an operating floppy disk drive voltage regulator. From [2], by permission. |
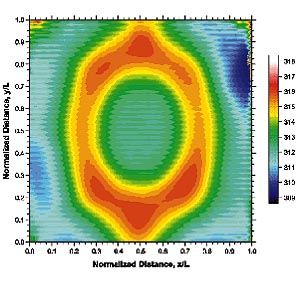 Figure 3. Temperature distribution under an array of slot jets impinging on a heatedsurface. From [3], by permission. |
What are TLCs?
At the heart of LC based thermography systems are liquid crystal materialscalled thermochromic liquid crystals (TLCs). Fundamentally, a liquid crystal isa thermodynamic phase that is between the pure solid and pure liquid phases ofmatter and exists in some organic compounds under certain conditions. Attemperatures below the TLC’s event temperature, a TLC will be in the solid stateand will appear transparent. When a TLC is at its event temperature, illuminatedby white light1 and viewed under fixed optical conditions, the TLCmaterial will reflect a unique wavelength2 of visible light (i.e.,color). As the temperature rises through the TLC’s bandwidth, the reflectedcolor of the TLC will change. Finally, when the temperature exceeds the TLC’sclearing point temperature, the material will enter the pure liquid state andwill revert back to being transparent. This phenomenon is selective reflectionand occurs in most TLCs both on heating and cooling and occurs with minimalhysteresis.
The molecular structure of a TLC gives the material two indices ofrefraction causing the material to become birefringent. Birefringence willcause the selectively reflected light emerging from a TLC to become circularlypolarized. The reflected color spectrum for most TLC materials will varycontinuously from the longer wavelengths (i.e., red) corresponding to the eventtemperature to shorter wavelengths (i.e., blue) corresponding to the clearingpoint temperature. Additionally, a TLC material will also transmit asignificant amount of the incident light with virtually no modification. Viewing TLCs against a non-reflecting, i.e., black, background prevents thistransmitted light from adversely affecting the interpretation of the selectivelyreflected light.
Classification of TLCs
The chemical makeup of a TLC material fixes its color-temperature responseat the time of manufacture. A simple, two color/temperature designatortypically describes this response and can be useful in qualitative applicationsand for properly selecting a TLC formulation for a particular application. Forexample, ‘R35C5W’ designates a commonly used TLC formulation. The ‘R35C’signifies that the red, ‘R’, start or event temperature of the TLC is 35°C. The ‘5W’ signifies that the blue start temperature is 5°C above the redstart temperature and this provides users with a crude estimate of theformulation’s active bandwidth. Narrow-band TLC formulations have bandwidthsbelow 1 or 2°C, while wide-band formulations typically have bandwidthsbetween 5°C and 20°C. “Off-the-shelf” TLC formulations areavailable with event temperatures ranging from -30°C to 100°C andbandwidths ranging from 0.5°C to 30°C.
Due to their inherently oily form, pure TLCs are very difficult to work withand their thermal performance degrades rapidly due to chemical contamination andexposure to ultra-violet radiation. To combat these effects, two manufacturingprocesses, microencapsulation and polymer dispersion, have emerged that attemptto protect the raw TLC material and to make them more usable.
Microencapsulation, (ME): Researchers at the National Cash RegisterCompany pioneered the development of microencapsulation techniques for TLCs inthe early 1970’s. Microencapsulation is a chemical process that takes raw TLCmaterial and encases it in protective 5-10 micron diameter capsules. Customtailored, sprayable formulations consisting of TLC-filled microcapsulessuspended in a water-based binder material are commercially available forvirtually any application. The ME process offers superb chemical contaminantresistance and good radiation protection and it can also make the TLC surfaceless sensitive to the lighting-viewing geometry. However, special care isnecessary to properly prepare and apply ME-TLCs in order to avoid problemsassociated with over-attenuation of the reflected light coming from the TLCs andsegregation of the binder and the micro-capsules.
Polymer dispersion, (PD): Researchers originally designed polymerdispersion methods for TLCs to facilitate the manufacture of continuous castingsand sheets of TLC material. The process chemically disperses pure monomer-basedTLC material into a solid polymer-based matrix. A key benefit of this processis that the virtually transparent polymer material only causes mild attenuationof the reflected light from the TLC. This feature preserves more of thebrilliant color response characteristics of the raw TLC material.
However, PD-TLCs are fairly limited in their usability for two reasons:
1) they are not typically available in a sprayable medium, limiting use torelatively flat surfaces;
2) edge effects due to chemical contamination can destroy a PD-TLC surfacewhen a cutout portion of a manufactured sheet is used.
Qualitative Temperature Visualization Techniques
Many temperature visualization applications only require qualitativeinformation. In these applications, the two color/temperature descriptors ofthe TLC material combined with the color response capabilities of our own eyescan readily provide a very simple solution. Qualitative TLC techniques aretypically easy and inexpensive to implement and can provide high spatialresolution when properly used in an application that provides suitable cellspacing=”0″ opticalaccess to the TLC coated surface. A typical qualitative TLC application couldprovide a quick investigation of an electronic component’s temperature responseto changes in thermal conditions like air flow rate and direction or componentorientation. Other qualitative TLC implementations may include verification ofisothermal and adiabatic temperature conditions in computer models ofelectronics cooling situations. Many quantitative measurement techniques thatuse the tristimulus (or three input) color response system of the human eye toinfer the TLC temperature have their origins in these types of qualitativemeasurement techniques.
Quantitative Temperature Measurement Techniques Using Narrow-Band andMulti-Event Narrow-Band TLC Formulations
Most quantitative LC thermography applications have used narrow-band ME-TLCformulations or multi-event temperature mixtures of several narrow-band TLCseach having different event temperatures. Collected data from successiveexperiments at different surface temperatures or power levels facilitatesconstruction of the surface isotherm patterns using single- or multi-eventformulations. Narrow-band TLC formulations can allow accurate verification ofthe surface temperature distribution of presumably isothermal objects. Thesmall range of valid temperatures in these formulations permits making highlyaccurate relative temperature measurements using narrow-band TLC formulationsand simple image processing systems. In situ color-temperature calibrations areusually sufficient to provide reasonably accurate absolute temperaturemeasurements with these formulations. On the down side, computations ofmulti-event isotherm patterns are usually very tedious and time-consuming andthe implementation of full-field measurement capabilities is difficult withnarrow-band formulations.
Using Wide-Band TLC Formulations
An alternative to using narrow-band TLCs is to use the wider bandwidth of asingle wide-band TLC to map the isotherm (i.e., isocolor) pattern of a surfacefrom a single image. These wide-band techniques are useful when an object haslarge temperature variations or when accurate measurement of an object’s surfacetemperature distribution warrants high spatial resolution. Practicalapplications of wide-band techniques are numerous. They include theinvestigation of the surface temperature distribution in such areas as gasturbine blade cooling, electronic component characterization and the study ofboiling heat transfer phenomena. Figures 2 and 3 show some examples of theseapplications.
The development efforts of LC thermography systems have shown that thefollowing issues are crucial when an application requires high measurementaccuracy from wide-band TLC formulations:
- Accurate and efficient descriptor of color that is usable in TLCapplications.
- TLC color-temperature response calibration.
- Lighting and viewing effects.
| Comparison of Narrow-Band and Wide-BandTechniques | ||
| Advantages | Disadvantages | |
| Narrow-band |
|
|
|
|
|
| Wide-band |
|
|
|
|
|
| table cellspacing=”0″ 1. Advantages anddisadvantages of using narrow- and wide-band TLC formulations in LC thermographysystems. | ||
Color descriptors: Since color is a subjective entity, it can bedifficult to quantify. However, many physiological studies have proved that therods and cones of the human eye decompose color into a combination of the red,green and blue (RGB) primary colors. Many modern machine vision systems haveimplemented this natural tristimulus decomposition as an attempt to emulate thehuman vision system. These systems store the appropriate amounts of red, greenand blue needed to produce a correspondingly matched color response at eachpoint in an image. However, this implementation is not very efficient for LCthermography because it requires that three values (red, green and blue, forexample) be used to interpret the temperature at each point in an LCthermograph. Furthermore, these RGB color measurement models alone aredifficult to use in applications that have non-ideal optical conditions present. However, recent development efforts have formulated very robust andcomputationally efficient single value color descriptors tailored specificallyfor LC thermography that are based on the standard RGB vision model, [5].
Color-temperature response calibration: Prior knowledge of theinput to output response characteristics of a measurement sensor is crucial toproper implementation and use of that sensor. In LC thermography applicationsthis means, ideally, that the intrinsic color-temperature response of the TLC beknown as well as any other inputs that may affect this response including themeasurement system itself.
The simplest way of doing this is to directly calibrate the TLC and colormeasurement system response characteristics in place, or in-situ. This methodrequires the apparatus under test be equipped with an external means ofcontrolling and measuring the TLC temperature at or near the surface area ofinterest. This method can be difficult or impossible to implement in someapplications and typically produces response characteristics that are not onlyspecific to the color measurement system and TLC, but to the actual applicationitself.
Alternatively, many researchers have focused on developing more systemindependent calibration methods with the intent of extracting the intrinsiccolor-temperature response of the TLC itself. These efforts have implementedsuccessive-isotherm and gradient techniques.
Successive-isotherm methods typically use a temperature controlled testsurface and a color measurement system to generate TLC color-temperaturecalibration data. Data acquisition commences by bringing the test surface andTLC to the TLC’s event temperature. Next, a color image of the surface is takenand an average color value is computed and stored with the temperature of thetest surface. This process is then repeated at subsequently higher temperaturesuntil the clearing point temperature is reached. Though seemingly simple toautomate, successive-isotherm methods can be very time consuming and typicallysuffer from poor color-temperature resolution and large amounts of required dataprocessing.
Gradient methods attempt to overcome many of the shortcomings ofsuccessive-isotherm methods by subjecting a TLC material to a measurablecolor-temperature distribution, such as a linear temperature gradient. Whenproperly implemented, a gradient technique can provide a continuous rather thandiscrete representation of the entire color-temperature response for a TLC usinga single color image. These gradient methods provide much highercolor-temperature resolution than successive-isotherm methods in a fraction ofthe time and with much less data processing.
Unfortunately, the development of these advanced calibration methods has notbeen able to completely eliminate the color measurement system from the TLCcolor-temperature calibration. However, due to their fundamental approach, theyhave significantly enhanced our understanding of many important issuesassociated with LC thermography.
Lighting and viewing: The perceived color of a pure TLC (at anyfixed, active temperature) is dependent on the lighting-viewing arrangement andon the amount of background and/or secondary light present. However, researchhas shown that using a co-aligned primary lighting-viewing system in the absenceof any background or secondary light, can minimize this dependency, [7]. Furthermore, the signal to noise ratio in these systems can be can be greatlyenhanced by cross polarizing the lighting-viewing system to maximize thetransmission of the circularly polarized light being reflected by the TLC.
Sources of Temperature Measurement Error
Temperature measurement error effects have been difficult to quantify in LCthermography applications. This is mainly due to the large number of possibleerror sources and the effects these sources have on the overall temperaturemeasurement accuracy of an LC thermography system. However, most measurementerrors can be attributed to one or more of the following effects:
- Improper surface preparation and/or application of the TLC material.
- Improper calibration and/or interpretation of the TLC color-temperature
- Fixed errors associated with the color and/or temperature measurementequipment of the system.
Summary
Many significant advances have arisen from a considerable amount offundamental and applied research and development of TLCs and LC thermographyover the past 20 to 30 years. These advances have led to much more stable cellspacing=”0″ andusable TLC formulations that cover a wide operating temperature range (15 to 100°C). Commercially available LC thermography systems have recently become availablewhich use color measurement models tailored to the complex responsecharacteristics of TLCs and state-of-the-art electronics technologies, in orderto deliver cost-effective and reliable temperature measurement performance. However, regardless of how impressive an LC thermography system may appear tobe, there is no replacement for the knowledge of how best to use the availableTLC technology.
The following set of ‘how-to’ guidelines should be helpful when consideringto use of LC thermography in an application:
- Determine the expected minimum and maximum temperatures of the surface(s)in question.
- Select an appropriate TLC for this temperature range: narrow-band,multi-event or wide-band.
- Properly prepare and apply the TLC material and black background to thetest surface.
- Provide adequate optical access to the surface and minimize unwantedreflections.
- Provide an on-axis lighting and viewing arrangement if possible withcrossed polarizers.
- Calibrate the color-temperature response of the TLC and imaging system ifquantitative measurements are needed.
- Image/visualize the active TLC surface.
- Relate color to temperature using the appropriate color-temperaturecalibration for the TLC.
Notes
- To be more precise, this wavelength of light is actuallythe dominant wavelength of a continuous wavelength spectrum and the dominantwavelength is of the most interest, [6].
- In this discussion white light denotes light that containsall the wavelengths of at least the visible portion of the electromagneticspectrum.
References
1. Azar, K., J. R. Benson & V. P. Manno (1991)Liquid Crystal Imaging for Temperature Measurement of Electronic Devices. IEEESemiconductor Thermal Measurement and Management Symposium, Phoenix, AZ, pp.23-33.
2. Batchelder, K. A. & D. J. Farina, Personal communication, August 9,1995.
3. Danek, C. J. & R. J. Moffat, (1995) Low Aspect-Ratio ImpingementCooling of Electronics with Turbulence Augmentation. Advances in ElectronicsCooling, proceedings of the ASME/JSME InterPack 95, Maui, HI, pp. 837-842.
4. Farina, D. J. & R. J. Moffat, A System for Making TemperatureMeasurements Using Thermochromic Liquid Crystals. Report Number HMT-48,Stanford University, Department of Mechanical Engineering, ThermosciencesDivision, Stanford, CA, September, 1994.
5. Hacker, J. M. & J. K. Eaton, Heat Transfer Measurements in aBackward Facing Step Flow with Arbitrary Wall Temperature Variations. ReportNumber MD-71, Stanford University, Department of Mechanical Engineering,Thermosciences Division, Stanford, CA, June, 1995.
Suggested Reading
6. Collings, P. J., Liquid Crystals: Nature’s DelicatePhase of Matter, Princeton University Press, Princeton, 1990.
7. Farina, D. J., J. M. Hacker, R. J. Moffat & J. K. Eaton, (1994)Illuminant Invariant Calibration of Thermochromic Liquid Crystals. ExperimentalThermal and Fluid Science, Vol. 9, No. 1, pp. 1-12.
8. Parsley, M. Hallcrest Handbook of Thermochromic Liquid CrystalTechnology Hallcrest, Inc., Glenview, IL, 1991.