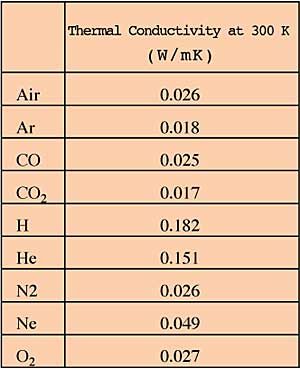
The value of thermal conductivity for most gases and vapors range between 0.01 and 0.03 W/mK at room temperature. Notable exceptions are Helium (0.15) and Hydrogen (0.18).
The most common theoretical explanation of heat conduction in gases is provided by the kinetic gas theory, which treats the collisions between the atoms or molecules as the prime mode of transfer of energy. Radiative heat transfer is neglected in this approach.
According to this theory, the thermal conductivity is proportional to the heat capacity per unit volume, the average gas velocity, and the mean free path. As the dynamic viscosity is proportional to the product of density, velocity and mean free path, it follows that the thermal conductivity is also proportional to the viscosity. In practice, the relationships are much more complicated and a high accuracy is not to be expected by deriving the thermal conductivity from the much more easily measured physical properties mentioned above.
With regard to the temperature dependency of the thermal conductivity, it is noted that its value increases roughly in proportion with the absolute temperature, at least in the range of ‘normal’ pressures. As pressure increases, so too does the thermal conductivity. At approximately 0.001 bar, the mean free path becomes of the same order as the walls confining the gas and the value increases linearly with pressure. Above 0.001 bar, the increase in thermal conductivity is of the order of magnitude of 1% per bar increase of pressure. From these figures, it can be concluded, for example, that changes in thermal conductivity due to atmospheric variations can be neglected in most cases.
The following table gives the thermal conductivity at room temperature for a variety of gases.