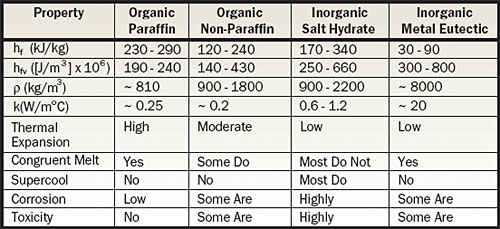
Table 1. General Solid-Liquid PCM Characteristics [1]
 |
When electronics are operated under transient conditions, increasing the thermal capacitance is a useful technique for limiting temperature increases and/or minimizing the performance requirements of a heat sink. One effective method of increasing thermal capacitance is to include a material that undergoes a change of phase at a desirable temperature. Examples of phase change that have been used in electronics cooling include solid-liquid, liquid-vapor, and solid-solid (e.g., crystalline structure to amorphous). A solid-liquid phase change is most common for systems that require reuse of the phase change material (PCM). The thermal energy required to melt the PCM is frequently described as the latent heat of fusion. Directing the waste heat from electronics into the PCM can result in a nearly isothermal heat sink while the PCM is melting.
Selecting a PCM for use in electronics cooling requires knowing the range of expected temperatures (the melt temperature of the PCM must be high enough such that melting does not occur until needed). Unfortunately, just knowing the desired melt temperature isn’t sufficient to select a PCM. There are hundreds of materials that melt in a temperature range useful for electronics cooling. However, the list of candidates becomes much smaller when issues such as material compatibility, toxicity, availability of thermal property data, and cost are considered.
Desirable characteristics of a solid-liquid PCM include high heat of fusion per volume, congruent melting and freezing characteristics, high thermal conductivity, minimal supercooling, and low thermal expansion. As is the case with most materials, searching for thermal property data will reveal some variability (and in many cases, complete thermal data doesn’t exist for some compounds). Table 1 lists four categories of solid-liquid PCMs with melting temperatures in the range of 40° to 120°C.
Looking at the table, it would appear that metal eutectic PCMs would be of interest but the main drawback is high density, resulting in heavy package solutions. Salt hydrates seem attractive until consideration is given to handling and safety issues. Two salt hydrates with high volumetric heat of fusion are Lithium Nitrate Trihydrate (679 x 106 J/m3) and Barium Hydroxide Octahydrate (656 x 106 J/m3). However, a search on material safety data sheets reveals that the first is a severe oxidizer with a moderate health rating and the second carries a severe health rating – these aren’t substances you would want to experiment with at home. The organic non-paraffin category includes a large number of candidate PCMs and has the largest variability within the table. Acetamide is an example of a non-paraffin organic PCM that melts at 81°C and has only moderate handling and material compatibility issues.
The organic paraffins represent a reasonable compromise between handling and performance and are available in a wide range of melt temperatures. In general, higher melt temperature paraffins are more expensive, especially for high purity levels. Low thermal conductivity of these materials usually requires the use of an imbedded matrix material to help conduct heat into the PCM. The volume change between solid and liquid states limits their packaging density.
References
- O’Conner, J., and Weber, R., “Thermal Management of Electronic Packages Using Solid-to-Liquid Phase Change Materials”, ISPS Proceedings, pp. 72 – 80, 1997.
- Hale, D.V., Hoover, M.J., and O’Neil, M.J., “Phase Change Materials Handbook”, NASA Contractor Report CR-61363, 1971.
- Humphries, W.R., and Griggs, E.I., “A Design Handbook for Phase Change Thermal Control and Energy Storage Devices”, NASA Technical Paper 1074, 1977.