Introduction
The cooling of electronic parts has become a major challenge in recent times due to the advancements in the design of faster and smaller components. As a result, different cooling technologies have been developed to efficiently remove the heat from these components [1, 2]. The use of a liquid coolant has become attractive due to the higher heat transfer coefficient achieved as compared to air-cooling. Coolants are used in both single phase and two-phase applications. A single phase cooling loop consists of a pump, a heat exchanger (cold plate/mini- or micro-channels), and a heat sink (radiator with a fan or a liquid-to-liquid heat exchanger with chilled water cooling) [3]. The heat source in the electronics system is attached to the heat exchanger. Liquid coolants are also used in two-phase systems, such as heat pipes, thermo-siphons, sub-cooled boiling, spray cooling, and direct immersion systems [2, 4].
Requirements for a Liquid Coolant for Electronics
There are many requirements for a liquid coolant for electronics applications. The requirements may vary depending on the type of application. Following is a list of some general requirements:
- Good thermo-physical properties (high thermal conductivity and specific heat; low viscosity; high latent heat of evaporation for two-phase application)
- Low freezing point and burst point (sometimes burst protection at -40°C or lower is required for shipping and/or storage purposes)
- High atmospheric boiling point (or low vapor pressure at the operating temperature) for single phase system; a narrow desired boiling point for a two-phase system
- Good chemical and thermal stability for the life of the electronics system
- High flash point and auto-ignition temperature (sometimes non-combustibility is a requirement)
- Non-corrosive to materials of construction (metals as well as polymers and other non-metals)
- No or minimal regulatory constraints (environmentally friendly, nontoxic, and possibly biodegradable)
- Economical
The best electronics coolant is an inexpensive and nontoxic liquid with excellent thermo-physical properties and a long service life. A high flash point and auto-ignition temperature are desired so that the fluid is less susceptible to ignition. Good thermo-physical properties are required to obtain the high heat transfer coefficients and low pumping power needed for the fluid to flow through a tube or a channel.
Electrical conductivity (not mentioned in the list) of a coolant becomes important if the fluid comes in direct contact with the electronics (such as in direct immersion cooling), or if it leaks out of a cooling loop or is spilled during maintenance and comes in contact with the electrical circuits [5]. In certain applications, a dielectric coolant is a must, whereas in many other applications it is not a requirement because of the very remote chance of coolant leakage (or in case of a leak, the coolant does not come in contact with the electronics).
Table 1: Properties of Different Liquid Coolant Chemistries at 20°C
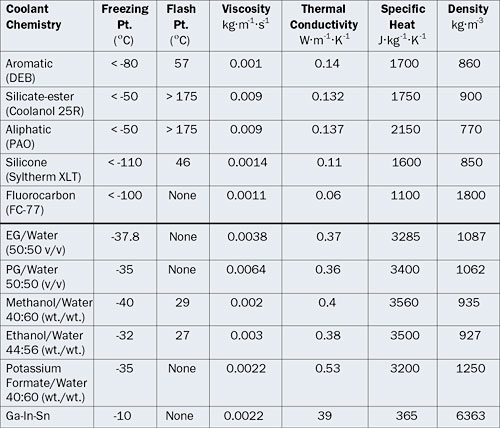 |
In the following sections, various liquid coolant chemistries are divided into dielectric and non-dielectric fluids and their properties are discussed in more depth (refer also to Table 1).
Dielectric Liquid Coolants
Aromatics: Synthetic hydrocarbons of aromatic chemistry (i.e., diethyl benzene [DEB], dibenzyl toluene, diaryl alkyl, partially hydrogenated terphenyl) are very common heating and cooling fluids used in a variety of applications [6]. However, these compounds cannot be classified as non-toxic. Also, some of these fluids (i.e., alkylated benzene) have strong odors, which can be irritating to the personnel handling them.
Silicate-ester: This chemistry (i.e., Coolanol 25R) was widely used as a dielectric coolant in Air Force and Navy airborne radar and missile systems. These fluids have caused significant and sometimes catastrophic problems due to their hygroscopic nature and subsequent formation of flammable alcohols and silica gel. Therefore, these fluids have been replaced by more stable and dielectric aliphatic chemistry (polyalphaolefins or PAO) [7].
Aliphatics: Aliphatic hydrocarbons of paraffinic and iso-paraffinic type (including mineral oils) are used in a variety of direct cooling of electronics parts as well as in cooling transformers [6]. Many petroleum based aliphatic compounds meet the Food and Drug Administration (FDA) and United States Department of Agriculture (USDA) criteria for incidental food contact. These petroleum-based fluids do not form hazardous degradation byproducts. Most of these fluids have a non-discernible odor and are nontoxic in case of contact with skin or ingestion. As mentioned before, aliphatic PAO-based fluids have replaced the silicate-ester fluids in a variety of military electronics (and avionics) cooling applications in the last decade.
Silicones: Another class of popular coolant chemistry is dimethyl- and methyl phenyl-poly (siloxane) or commonly known as silicone oil [6]. Since this is a synthetic polymeric compound, the molecular weight as well as the thermo-physical properties (freezing point and viscosity) can be adjusted by varying the chain length. Silicone fluids are used at temperatures as low as -100°C and as high as 400°C. These fluids have excellent service life in closed systems in the absence of oxygen. Also, with essentially no odor, the non-toxic silicone fluids are known to be workplace friendly. However, low surface tension gives these fluids the tendency to leak around pipe-fittings, although the low surface tension improves the wetting property. Similar to the aliphatics, high molecular weight silicone oils have also found applications in cooling transformers.
Fluorocarbons: Fluorinated compounds such as perfluorocarbons (i.e., FC-72, FC-77) hydrofluoroethers (HFE) and perfluorocarbon ethers (PFE) have certain unique properties and can be used in contact with the electronics [4, 8]. First of all, these fluids are non-combustible and non-toxic. Some fluorinated compounds have zero ozone depleting potential and other environmental properties. Secondly, some of these fluids have low freezing points and low viscosities at low temperatures. However, these fluids are very expensive, have poor thermal properties, some of them have global warming potential (greenhouse effect), and, due to the extremely low surface tension, leaks can develop around fittings.
Non-Dielectric Liquid Coolants
Non-dielectric liquid coolants are often used for cooling electronics because of their superior thermal properties, as compared with the dielectric coolants. Non-dielectric coolants are normally water-based solutions. Therefore, they possess a very high specific heat and thermal conductivity [9]. De-ionized water is a good example of a widely used electronics coolant. Some other popular non-dielectric coolant chemistries are discussed below:
Ethylene Glycol (EG): Commonly used as antifreeze in automotive engine cooling, EG also has found use in many industrial cooling applications. Common applications include process cooling at lower temperatures. Ethylene glycol is colorless and practically odorless and is completely miscible with water. When properly inhibited, it has a relatively low corrosivity. However, this coolant is classified as toxic and should be handled and disposed of with care. The quality of water used for the preparation of a glycol solution is very important for the system. Typically, water with low chloride and sulfate ion concentration (< 25 ppm) is recommended. Also, a monitoring schedule should be maintained to assure that inhibitor depletion is avoided and pH of the solution is consistent. Once the inhibitor has been depleted, it is recommended that the old glycol be removed from the system and a new charge be installed.
Propylene Glycol (PG): In its inhibited form, PG has the same advantages of low corrosivity shown by ethylene glycol. In addition, propylene glycol is considered non-toxic. Other than lack of toxicity, it has no advantages over ethylene glycol, being higher in cost and more viscous.
Methanol/Water: This is a low cost antifreeze solution, finding use in refrigeration services and ground source heat pumps. Similar to glycols, this can be inhibited to stop corrosion. This fluid can be used down to -40°C owing to its relatively high rate of heat transfer in this temperature range. Its main disadvantages as a heat transfer fluid are its toxicological considerations. It is considered more harmful than ethylene glycol and consequently has found use only for process applications located outdoors. Also, methanol is a flammable liquid and, as such, introduces a potential fire hazard where it is stored, handled, or used.
Ethanol/Water: This is an aqueous solution of denatured grain alcohol. Its main advantage is that it is non-toxic. Therefore, it has found application in breweries, wineries, chemical plants, food freezing plants, and ground source heat pumps. As a flammable liquid, it requires certain precautions for handling and storage.
Calcium Chloride Solution: Aqueous solutions of calcium chloride find wide use as circulating coolants in food plants. It is non-flammable, non-toxic and thermally more efficient than the glycol solutions. A 29% (by wt.) calcium chloride solution has a freezing point below -40°C. The main disadvantage of this coolant is that it is highly corrosive even in the presence of corrosion inhibitors.
Potassium Formate/Acetate Solution: Aqueous solutions of potassium formate and acetate salts are non-flammable and non-toxic as well as much less corrosive and thermally more efficient than calcium chloride solution [5]. Therefore, even with higher price than calcium chloride, they have found a large number of applications in recent years. Although the main applications of these fluids are in the food, beverage, pharmaceuticals, chemical and climatic chamber applications, recently these fluids have been investigated for single-phase convection cooling of microprocessors.
Liquid Metals: Recently, liquid metals of Ga-In-Sn chemistry have been utilized with a magnetofluiddynamic (MFD) pump [2]. It utilizes the high thermal conductivity and density of the metal alloy to remove very high heat flux from microprocessors.
Other Exotic Coolant Chemistries
Other than the chemistries discussed above, there are some new developments in the liquid coolant field. Nanofluids (dispersions of nanoparticles such as metal oxide, metal, carbon nanotube, or diamond in a coolant to increase thermal conductivity) have been investigated as a method to improve thermal performance of existing chemistries [10].
The number of journal publications in this field has increased exponentially in the recent years. However, there are still a large amount of unknown factors (i.e., long-term reliability, agglomeration, settling, and blockage of micro-channels) existing in the utilization of nanoparticles in a coolant. Phase change materials (PCM) in their micro- or nano-encapsulated form have been utilized in a coolant medium to increase the specific heat capacity. Again, reliability has been a problem in utilizing them.
Ionic liquids (room temperature liquid salts) have also shown some potential to become next-generation coolants based on their thermal stability, extremely low vapor pressure and other properties. Currently, their application is limited to solvents in chemical reactions and extractions. These chemistries will take a number of years to become technically and economically competitive with the existing coolants.
Conclusions
There are several coolants (both dielectric and non-dielectric) commercially available. However, selection of the best coolant for a particular application requires a proper understanding of all the characteristics and thermo-physical properties of these fluids. Dielectric fluids can be used in contact with the electronics, whereas non-dielectric coolants are used with a cold plate. In the future, coolants with better properties (thermal conductivity, specific heat, thermal stability) may be available, but their popularity will depend on their reliability and economics.
References
- Incropera, F., Liquid Cooling of Electronic Devices by Single-Phase Convection, New York: John Wiley & Sons, 1999, pp. 1-14.
- Lasance, C. and Simons, R., “Advances in High Performance Cooling for Electronics,” ElectronicsCooling, Vol. 11, No. 4, 2005, pp. 22-39.
- Schmidt, R., “Liquid Cooling is Back,” ElectronicsCooling, Vol. 11, No. 3, 2005, pp. 34-38.
- Chrysler, G. M., Chu, R., and Simons, R.E., “Jet Impingement Boiling of a Dielectric Coolant in Narrow Gaps,” IEEE Transactions CHMT-Part A., Volume 18, No. 3, 1995, pp. 527-533.
- Mohapatra, S. and Loikits, D., “Advances in Liquid Coolant Technologies for Electronics Cooling,” Proceedings of the 21st IEEE Semiconductor Thermal Measurement and Management Symposium, San Jose, CA, 2005, pp. 354-360.
- Mohapatra, S., “Select Heat Transfer Fluids for Low-Temperature Applications,” Chemical Engineering Progress, August 2001, pp. 47-50.
- Ghajar, A., Tang, W. and Beam, J., “Comparison of Hydraulic and Thermal Performance of PAO and Coolanol 25R Liquid Coolants,” 6th AIAA/ASME Joint Thermophysics and Heat Transfer Conference, Colorado Springs, CO, June 20-23, 1994, pp. 1-14.
- Maddox D.E. and Mudawar, I., “Critical Heat Flux in Subcooled Flow Boiling of Fluorocarbon Liquid on a Simulated Electronic Chip in a Rectangular Channel,” International Journal of Heat and Mass Transfer, Volume 32, 1989, pp. 379-394.
- ASHRAE Handbook Fundamentals, Atlanta: American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc, 2001.
- Marquis, F. and Chibante, L., “Improving the Heat Transfer of Nanofluids and Nanolubricants with Carbon Nanotubes”, JOM, 57 (12), pp. 32-43, 2005.