This column deals with the thermal conductivity and melting points of liquid metals. Thinking about liquid metals, many people associate them with high temperatures. It is not widely known that some metallic alloys are liquid below 0�C, with the exception of course of mercury which was already known in ancient times. China’s first emperor is said to have been buried in rivers of flowing mercury.
In the field of thermal management we are interested in liquid metals at or around room temperature for two main purposes: for reducing thermal interface resistances and for liquid cooling. Both applications increase in importance because they enable significant reductions in the overall thermal resistance. Except for the much higher thermal conductivity compared to ‘standard’ heat transfer fluids, liquid metals offer the additional advantage of having the ability to pump more efficiently due to their low electrical resistivity. At higher temperatures the most important industrial application is soldering. For an overview of thermal data see [1]. Other important applications at still higher temperatures are the use of Na for heat pipes and for the nuclear power industry.
From a solid-state-physics point of view, liquid metals are easier to deal with than solid metals because tensors transform to scalars due to the isotropy of the liquid state. This leads to a simple correlation between the electronic contribution to the thermal conductivity kel, the electrical resistivity and the temperature, the so-called Wiedemann-Franz law (see also [2]). Because the lattice contribution is usually two orders of magnitude smaller than the electronic contribution, it is safe to conclude that the thermal conductivity calculated by using the W-F law is a reasonable estimate of k, the advantage being that r is much easier to measure.
Focusing on pure metals, all alkalines have low melting points. Unfortunately, all of them are very reactive with many fluids and most metals, are sometimes toxic, and hence ceramic coatings are often mandatory. Most alloys for low melting points consist of a mixture of Ga, In, Sn, Pb and Bi, leading to a whole range of eutectics. Of particular importance is a GaInSn combination sold as Galinstan that is accepted as a replacement of mercury.
Table 1. Thermal Conductivity and Melting Points of Some Metals and Alloys
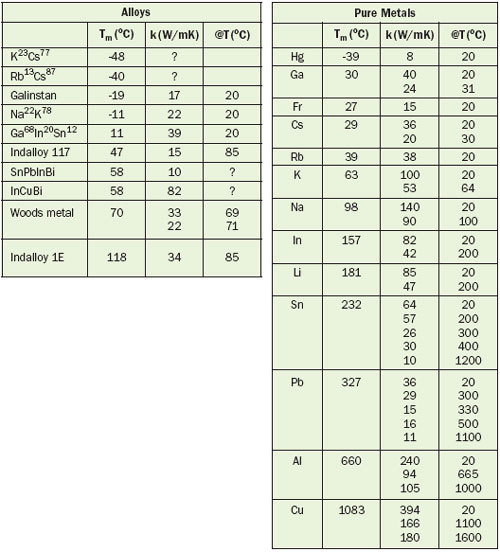 |
The table shows the thermal conductivity and melting points for a selection of pure metals and alloys. Many non-eutectic alloys don’t have a well-defined melting point but rather a melting range, and hence data for melting points are indications. Watch the sharp drop in k from solid to liquid, caused by the fact that the crystalline order disappears during the phase change leading to many more scattering modes. Rule-of-thumb: k is about halved.
References
- Technical Data, ElectronicsCooling, Vol. 12, No. 3, August 2006.
- Technical Data, ElectronicsCooling, Vol. 6, No. 2, May 2000.