Removing waste heat from electronic assemblies using a circulating liquid coolant is a common and effective method with a long and successful history, especially in avionics and data center applications. The choice of coolant depends on many factors including corrosion resistance, cost, thermal properties, regulatory constraints, thermal stability, and environmental temperature considerations [1]. One figure of merit for comparing heat transfer performance of coolants is the Mouromtseff number [2]. In some instances, the dielectric property of the coolant is important. For these applications coolants like polyalfaolefin (PAO), silicon oil, and fluorocarbons are used, but in general, their thermal conductivity and specific heat values are low. As a coolant, water fulfills many of the desirable thermal characteristics, but for liquid cooling systems that are subject to environmental temperatures below 0�C, the need for freeze protection precludes using just water.
The most common water based antifreeze solutions used in electronics cooling are mixtures of water and either ethylene glycol (EGW) or propylene glycol (PGW). The use of ethylene glycol has a longer history, especially in the automotive industry. However, EGW solutions formulated for the automotive industry often have silicate based rust inhibitors that can coat and/or clog heat exchanger surfaces. The use of PGW as a coolant is becoming more common primarily because it is environmentally friendly and non-toxic. Ethylene glycol is listed as a toxic chemical requiring care in handling and disposal. The thermal properties of EGW and PGW solutions are similar but there are differences to note, especially for thermal engineers familiar with EGW but now designing with PGW. In general, for the same level of freeze protection, the thermal performance of PGW will be less than EGW. An additional difference is that a minimum freezing point for an EGW solution occurs with about a 63/37 volumetric ratio of ethylene glycol to water, but the freezing point of PGW continues to decrease as the percentage of propylene glycol increases. For applications requiring coolant flow in low temperature conditions, such as starting from a cold temperature, the viscosity of PGW is higher and significantly more pumping power is required.
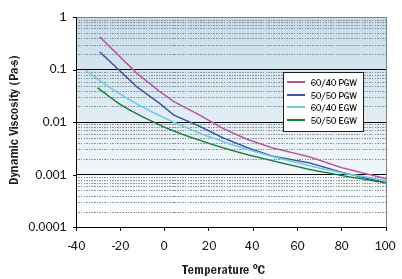 |
Figure 1. Viscosity variation with temperature for EGW and PGW solutions.
Figures 1 through 3 illustrate the viscosity, specific heat, and thermal conductivity variation with temperature for two volumetric ratios each of water/ethylene glycol and water/propylene glycol. These data are representative and specific manufacturers’ data should be consulted for specific values. Note that even though the density of these solutions decrease with increasing temperature, the volumetric heat capacity (density multiplied by specific heat) will increase slightly with increasing temperature. In particular, data for thermal conductivity variation with temperature are not consistent and some manufacturers indicate decreasing thermal conductivity with increasing temperature at concentrations greater than 50 percent. The trend of thermal conductivity variation with temperature shown in Figure 3 is consistent with reference 3. Most readily available formulations of EGW and PGW contain corrosion inhibitors that affect the physical properties of the solution.
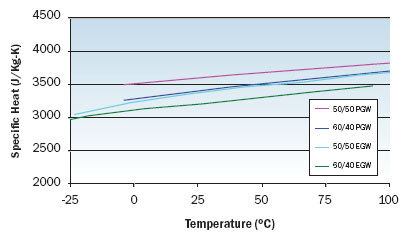 |
Figure 2. Specific heat variation with temperature for EGW and PGW solutions.
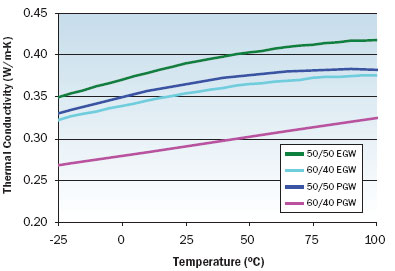 |
Figure 3. Thermal conductivity variation with temperature for EGW and PGW solutions.
References
- Mohapatra, S., “An Overview of Liquid Coolants for Electronics Cooling,” ElectronicsCooling, Vol. 12, No.2, May 2006.
- Simons, R., “Comparing Heat Transfer Rates of Liquid Coolants Using the Mouromtseff Number,” ElectronicsCooling, Vol. 12, No.2, May 2006.
- Assael, M.J., Charitidou, E., Avgoustiniatos, S., Wakeham, W.A., “Absolute Measurements of the Thermal Conductivity of Mixtures of Alkene-Glycols with Water,” International Journal of Thermophysics, Vol. 10, Issue 6, Nov. 1989.