Horror vacui, Natura abhorret vacuum, Resintenza del vacuo; from Aristotle to Galileo, it has long been known that here on earth matter tends to flow from where it is to where it isn’t. Fast forward to Hobbes and Boyle, Newton and Leibniz and we find such concepts being applied in the context of a fluid dynamic. From a heat transfer perspective heat energy also tends to flow from where it is to where it isn’t, from where it is hot to where it is cold. An equilibrium will be reached. As humans we love energy, we can do lots of interesting things with it. We constantly endeavor to get all those Joules to do our bidding, including storing them for later use, which in many ways can be like trying to corral cats.
So it took a couple of hours to heat (a point in) the water tank to 60degC. As sure as eggs those Joules aren’t going to hang around. They will leak out and in their wake, they will leave the water at the same temperature as the ambient in which the water tank sits. Let’s take a qualitative look at how insulation surrounding the tank plays a role in delaying the inevitable.
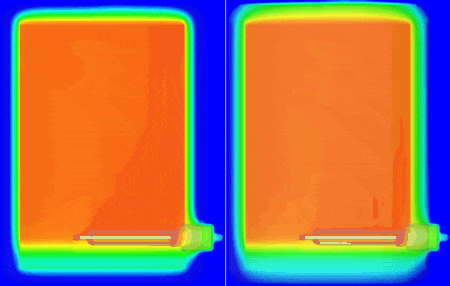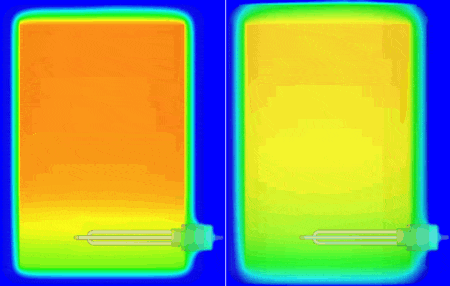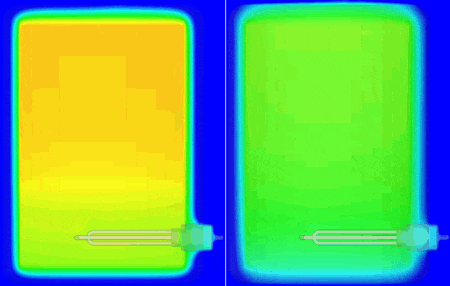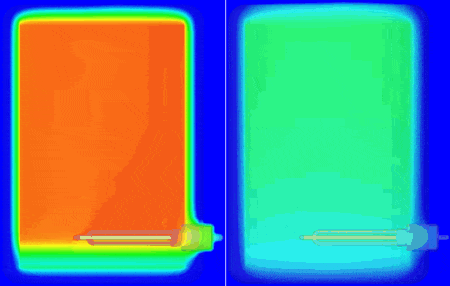
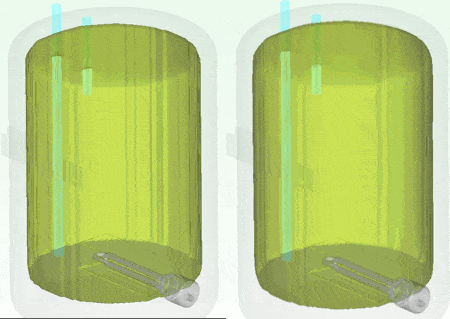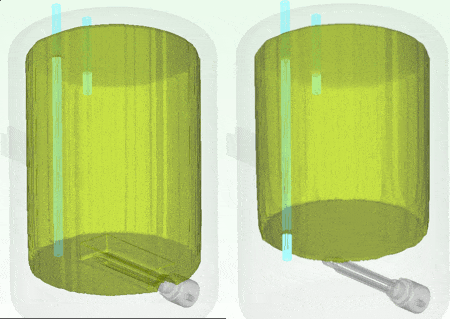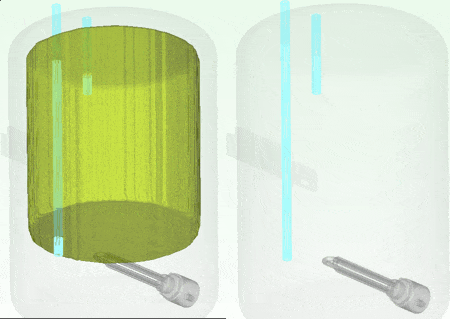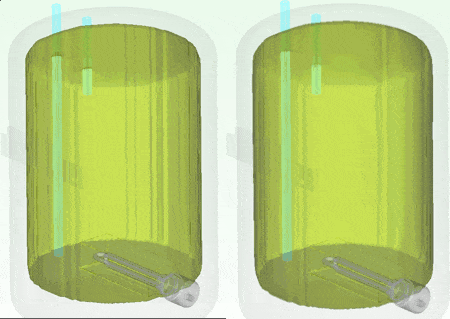
I personally don’t have a material thermal conductivity tester to hand (but sure, we do offer one in the form of our DynTIM). So far in this study I’ve assumed the insulation on my tank is expanded polyurethane type foam with a very low conductivity of 0.03 W/mK, that’s about 6000 times better at keeping the heat in compared to Aluminium. What if it wasn’t as good an insulator? What if it was say, 0.24 W/mK about that of cotton. Let’s compare and contrast how the heat leaves and the water cools when using these different insulation materials. Good insulation on the left, not so good on the right, the temp range is from 25 to 62 degC. The simulation simply carried on from the end of the warm-up, but with the power to the heater element turned off and the resulting change in temperature simulated over the next 10 hours:
The water at the top of the boiler certainly remains hotter for longer which is why the hot water extract pipe is always higher up in the tank. Seen even more clearly if we look at how the volume of water that is greater than (an arbitrary) 54.5 degC shrinks over that time:

9th January 2014, Ross-on-Wye