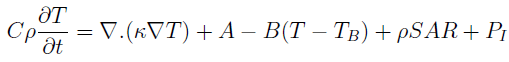We are entering an era where electronics technology is increasingly being integrated with biological systems in vivo. These technology solutions interact with organs and skeletal-, muscular- and nervous-systems of biological entities through hybridization of disparate solutions that merge to serve a common purpose. Biohybrid systems that interface and thus enable the interaction between biological systems and technology represent transdisciplinary efforts to address a common problem. In this setting, thermal management takes a very prominent role when engineers develop new implantable electronics solutions.
There’s a long history of embedding technology solutions inside a human body, starting back in 1958 with the invention of implantable pacemakers. The first few prototypes were short-lived and had to be replaced after a few hours. Today the technology has matured to a level where their form factors have shrunk and their batteries operate reliably for 15 years or more. Regulating bodies like the FDA make sure the implantable / embedded devices operate reliably and meet stringent approval requirements. The implantable electronics solutions now being developed include a wide spectrum of products ranging from smart phones to ingestible pills.
The merger of electronics in biological entities is not without problems. One of the major requirements is the biocompatibility –all implantable devices, including wearable electronics, that come in contact with the tissues of internal organs and/or skin must not cause any adverse reaction in the biological entity. In addition, the biocompatible materials must be stable over time and with temperature variations in the physiological environment where they function. They must be free of Cancer-causing toxins. The insulation materials must match the texture and smoothness of the tissue or the subdermal skin surfaces they interface with. The surfaces of the embedded electronics must also be chemically inert to variations in pH, and they should match the thermo-mechanical behavior of human tissue. Unless intended by design, implanted devices must also be hermetically packaged so as not to allow entry of body fluids nor allow the electronic hardware’s internal components to leach into the surrounding tissue.
Public perception of the emerging electronic bioimplants is nascent at best but it is improving when compared to a few years ago when apprehensions on their perceived harmful effects outweighed their utility. With the exception of Cardiac Pacemakers, which are well-known and more recently implantable insulin pumps, newer implants like the early subdermal RFID chips were met with suspicions. VeriChip, for example, introduced implantable microchip/RFID, which received United States FDA approval in 2004. When the implanted chip is scanned at the proper frequency, VeriChip outputs a unique number which can be linked with information about the user held in a database for ID verification, accessing medical records, etc. However, when lab mice and rats were implanted with RFID transponders like those made by VeriChip, some developed malignant and lethal cancers where 1% to 10% of cases were found at the site of the microchip. Though these studies could not be proven for human subjects, the lab results were enough to sink VeriChip’s stock.
Challenges in Thermal Management of Bioimplants
Implantable electronic components require space, power to operate, and will generate waste heat. Therefore, the component size, power consumption, material sets, packaging techniques, and thermal management become more important than before in the design of implantable electronics.
In addition to the foregoing parameters that are common to all electronics applications, the following are some of the unique challenges when it comes to thermal management of bioimplants:
- thermal properties of biomaterials (a good source of data can be found here)
- effect of blood flow which has significant quantitative effect on temperature distribution
- interfacial contact resistance
- varying effects of temperature on tissue types
- communication duty cycles of embedded electronic components
A good synopsis of thermal effects of bioimplants can be found in this article, which delves into the effects of various parameters on the temperature increase in human body tissue.
Most implantable electronic components with complex electronic circuitry operate well within power dissipation and thermal limits of the human threshold. However, in applications where the implant constantly stimulates the surrounding tissue and communicates with the external world, thermal dissipation and its management has the potential to be an important element of the design. In some cases, the thermal dissipation can lead to temperature increases in the human tissue that are no longer negligible. In addition, there may be stringent limits on specific absorption limits (SAR) and maximum permissible exposure (MPE) in cases where the implanted components communicate with external sources at frequencies greater than 100 kHz.
The effect of blood perfusion and the SAR can be considered in the Fourier equation seen below (modified Pennes equation):
where C is the specific heat, r is the mass density of the material, k is the thermal conductivity, T is the temperature, A is the rate of heat production due to metabolic processes, B is the blood perfusion constant which quantifies the amount of blood flow to the tissue under consideration, and TB is the temperature of the blood which flows through the tissues.
Application Example: Macular Degeneration and Retinal Implants
Macular Degeneration is a class of degenerative diseases that results in progressive loss of central vision, typically in adults age 55 or higher. It affects the macula, or center of the retina. Though it does not result in complete loss of vision, degeneration of central vision can make it harder to read, drive, or function normally in activities of daily life.
This infographic on facts and figures about Macular Degeneration estimates that by 2020, there will be 200 million people worldwide with visual impairments, 40 million of which will be from advanced age-related Macular Degeneration (AMD).
While researching for this blog, I ran into an interesting case study about an artist who is an active painter even after being afflicted with AMD. You can read about the Macular Degeneration of Marion Gurfein’s eyes on her page at BrightFocus. Shown below are two of her paintings, one of which was completed 4 years after her diagnosis (Source).

In order to understand how retinal implants help to address Macular Degeneration, it is useful to review the anatomy of the human eye, shown below.
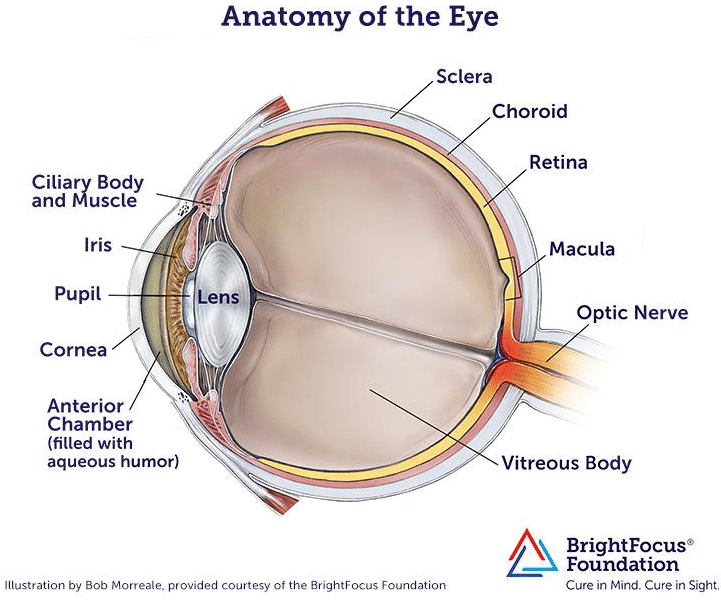
Retinal implants introduce visual information to the retina by electrically stimulating the surviving retinal neurons and restoring vision in blind people. This is accomplished by capturing images of the field of vision with a digital camera, processing these images into electronic data signals for transmission and then sending them by wireless transmission to a receiver implanted in the eye. This receiver translates these data signals into electric stimulation currents which are applied through microelectrodes to the epiretinal side of the eye. This leads to an activation of the underlying nerve cells that eventually elicit visual perceptions in the brain.
The retinal implant system by IMI, for example, consists of three main components:
- Implantable Retina Stimulator
- Visual Interface
- Pocket Processor
The Visual Interface (which resembles a bulky eye glass) and the Pocket Processor (about the size of a smaller smart phone) are external components. The accompanying software allows the user to tune certain parameters to meet the needs of individual patients.
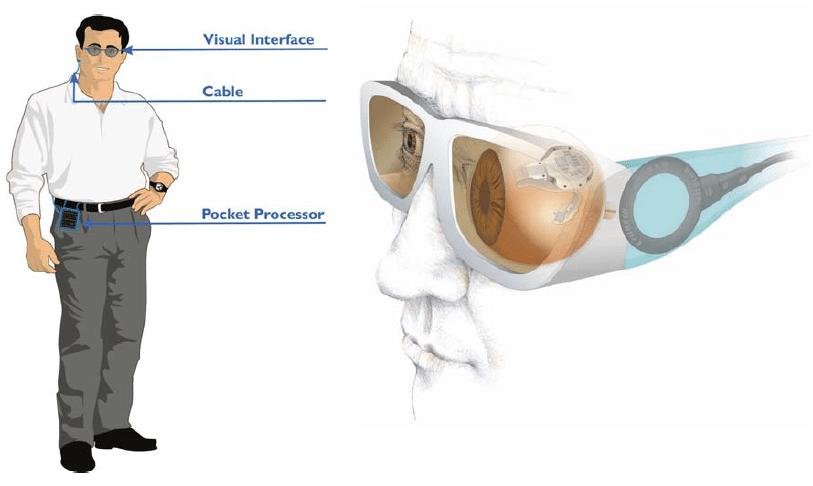
The Visual Interface (eye glasses) features a digital camera and transmitters for data and energy. It is connected to the Pocket Processor via a cable. The camera in the Visual Interface features a dynamic range, vision-capable for both indoor and outdoor environments. The power supply is in the Pocket Processor.
The processed visual information is sent by the Pocket Processor to an IR transmitter in the Visual Interface. The processed and transmitted visual data stream is then received by the IR receiver on the implant in the eye via IR light-emitting diodes.
Three types of retinal implants are common: epiretinal (on the retina), subretinal (behind the retina), and suprachoroidal (between the choroid and the sclera). They are depicted in the figure below.
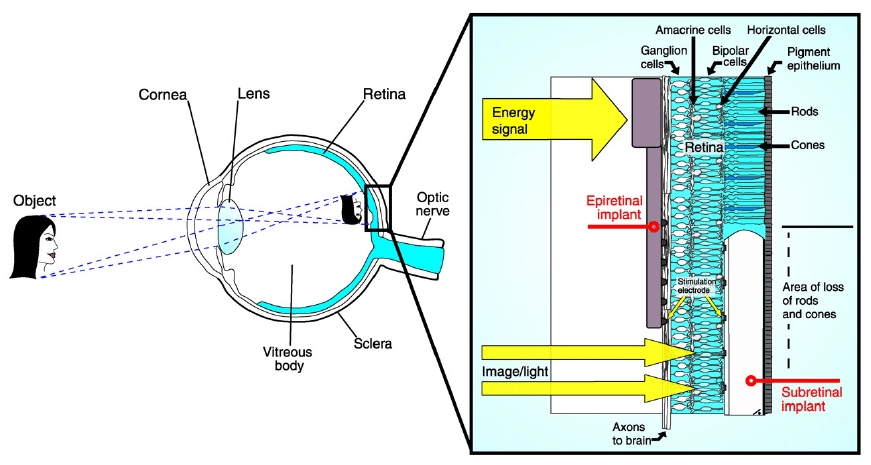
Retinal implants generate electromagnetic fields in the human head due to active telemetry with primary coil in the external Visual Interface and the secondary coil implanted in the retina. In the following, some results from a 60-electrode retinal prosthetic device dissipating 97mW of power from a dissertation at North Carolina Sate University are presented.
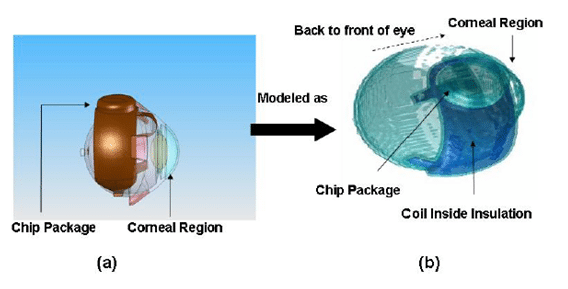
The simulation employed a finite difference scheme combining the explicit and the Alternating-Direction Implicit (ADI) method to solve the bioheat equation for different placements of the implant. The simulations included the basic model of the human head in 1-mm resolution slices from the Visible Man Project from the National Library of Medicine. The eye model was further refined and incorporated into the head model for accuracy.
The following figure shows the model used in the simulation described in the dissertation.
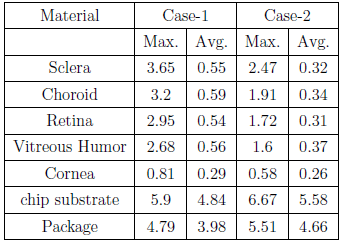
The simulation first establishes the initial temperature distribution in the human head for an initial temperature equal to the ambient temperature of air. Subsequently, the model was refined to obtain the initial reference temperature which is then used in the simulation of the temperature increase due to the implant. Note that the simulation evaluates the case where the implant is positioned on the sclera with a finite gap between the implant and the scleral tissue. Case #1 with no gaps represents the worst case since there will be maximum transfer of heat to the retinal tissues. Case #2 assumes a gap of 0.5mm. The following temperature increments (in degC) were observed in implant materials and key tissues due to operation of chip:
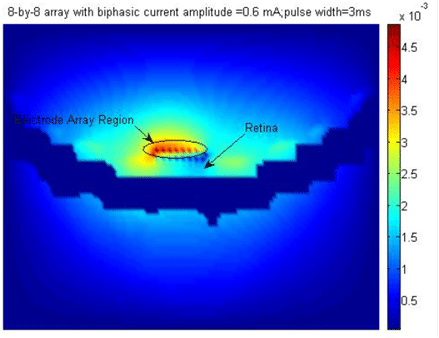
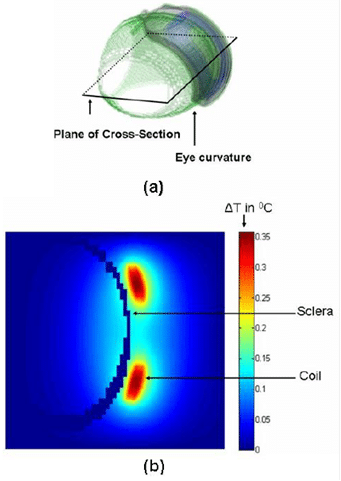
Localized thermal effects from retinal implants can be damaging to retinal tissue and thermal simulations help validate the implant design. The thresholds for thermal damage to normal tissues including the retina are dependent on a metric known as Cumulative Equivalent Minutes at 43°C (CEM43). Data is available to assess the damage based on an empirical equation for CEM which is a function of the exposure time, average temperature and a constant.
Retinal implants show good promise to those afflicted with eye diseases like Macular Degeneration. However, to date, only one retinal implant has received FDA approval. More are under development.
In closing, my blogs to date are always technology-focused and I seldom address the sentimental side of the subject matter. However, this blog is an exception. After reading about Marion Gurfein, I couldn’t help but admire her tenacity and her determination to forge ahead in life, refusing to be defined or limited by a disease like Macular Degeneration. And it makes me say this with pride: if I have seen this world in a better light, it is through the eyes of fighters like Marion Gurfein. And it also makes me invoke one of Sir Isaac Newton’s best-known aphorisms: If I have seen further it is by standing on the shoulders of Giants. She may not know it yet, she is a giant in a league of her own!
Your comments are always welcome.
by MP Divakar, PhD, Stack Design Automation
Technical Editor, Electronics Cooling